ELICIT Consensus Meeting
Maddie Clout, a trial manager from the BTC, recently attended a consensus meeting for the development of a core outcome set relating to the evaluation of interventions for informed consent for randomised controlled trials (ELICIT). Core outcome sets define a group of outcomes which all trials in a certain research area should collect, allowing the results of those trials to be more easily compared. The meeting was held at the University of Aberdeen and funded by the Medical Research Council.
The group of triallists, statisticians and members of the public met following a previous literature review and Delphi process, which had identified and ranked 43 potential outcomes across themes including the experience of decision-making and participant characteristics. The group worked together to reach a consensus on which of these 43 items should form the final core outcome set, and so be collected by all future trials in this research area. Maddie said: “It was fascinating to hear insights from the diverse group, and see areas in which triallists and patients ranked the importance of outcomes very differently from each other!” Fuelled by coffee and pastries, the group came to a consensus and a final list of outcomes was agreed upon, which will be published in the near future.
If you want to find out more about core outcome sets, the COMET Initiative have produced an animation.
FITNET-NHS: the biggest Paediatric Chronic Fatigue Syndrome/Myalgic Encephalomyelitis (CFS/ME) Trial
 Most UK children with Chronic Fatigue Syndrome (CFS/ME) have no access to local specialist treatment. FITNET-NHS tests the delivery of specialist one-to-one home treatments via the Internet, bringing treatment to families across the UK without the need for travel. All children in the trial are offered one of two treatments from a specialist at the Bath Paediatric CFS/ME Service:
Most UK children with Chronic Fatigue Syndrome (CFS/ME) have no access to local specialist treatment. FITNET-NHS tests the delivery of specialist one-to-one home treatments via the Internet, bringing treatment to families across the UK without the need for travel. All children in the trial are offered one of two treatments from a specialist at the Bath Paediatric CFS/ME Service:
- FITNET-NHS, a Cognitive Behavioural Therapy (CBT) program – delivered online
- Activity Management – delivered via Skype
The FITNET-NHS Trial has now become the world’s biggest Paediatric Chronic Fatigue Syndrome/Myalgic Encephalomyelitis (CFS/ME) Trial. The FITNET-NHS trial has recruited 246 participants and we aim to recruit a total of 314 participants by the end of October 2020.
The study is led by Prof Esther Crawley and the research team (Dr Emma Anderson, Mrs Manmita Rai and Dr Roxanne Parslow) who are all based at The Centre for Academic Child Health, Population Health Sciences, University of Bristol. The study is funded by the National Institute of Health Research (NIHR) and is sponsored by the University of Bristol. You can find out more information on the FITNET-NHS program website.
The PURSUIT study is now open to recruitment!
Proper Understanding of Recurrent Stress Urinary Incontinence Treatment in women: a randomised controlled trial of endoscopic and surgical treatment
The PURSUIT study is a NIHR-HTA funded (17/95/03) two-arm trial randomising women with recurrent, or persistent, stress urinary incontinence (SUI) to endoscopic urethral bulking injections or a surgical operation. There is currently no good evidence about which is the most effective treatment for improving symptoms and quality of life in women with this condition. We plan to recruit 250 participants from 24+ NHS hospitals throughout the UK and the study will run for six years.
Latest News
- Our first three pilot sites open to recruitment (North Bristol NHS Trust, Birmingham Women’s and Children’s NHS Foundation Trust and NHS Ayrshire & Arran) have all opened to recruitment in the last six weeks.
- Two further sites are due to open in early March completing our target of five pilot sites.
- North Bristol NHS Trust have recruited our first two participants to the study – fantastic work!
- In April and May we will be training 19 new NHS sites to join the main phase of the study, bringing our total number of recruiting sites to 24.
For further info
You can find out more information on the PURSUIT study website.
Email: pursuit-trial@bristol.ac.uk
Twitter: @PursuitTrial
Trial Manager: Dr. Caroline Pope
Trial Administrator: Miss Lucy Clark
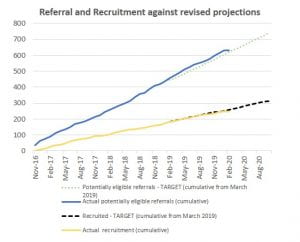
The first SISMIC Investigators’ meeting was held on 20th February 2020
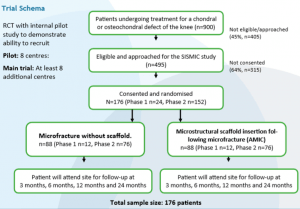
Up to 10,000 symptomatic articular cartilage injuries occur in the UK each year and treatments aim to either restore or replace the articular cartilage. Cartilage restoration often uses microfracture with or without scaffold insertion. Microfracture involves penetrating the subchondral bone in the area of injury to release fibrin and stem cells, which leads to the development of cartilage with scar tissue. It has been suggested that results may be better if a scaffold is used to help the repair.
The SISMIC study will investigate the clinical and cost-effectiveness of microstructural scaffold insertion following microfracture versus microfracture alone for the treatment of patients with chondral knee defects.
The Investigators’ meeting incorporated cadaveric training which was funded and provided by Joint Operations (one of the scaffold suppliers). Surgeons were able to practice the techniques which will be used in the study. Representatives from Baxter and Conmed attended and provided training on the use of the Tisseel glue which can be used in place of sutures to fix the scaffold in place. The meeting also allowed investigators from sites to meet with the wider research team.
This study is funded by the National Institute for Health Research-Health Technology Assessment Programme (NIHR127849). The views expressed are those of the author(s) and not necessarily those of the NHS, the NIHR or the Department of Health and Social Care.
You can find out more information on the SISMIC study website.
Email: sismic-study@bristol.ac.uk
Study Manager: Holly McKeon